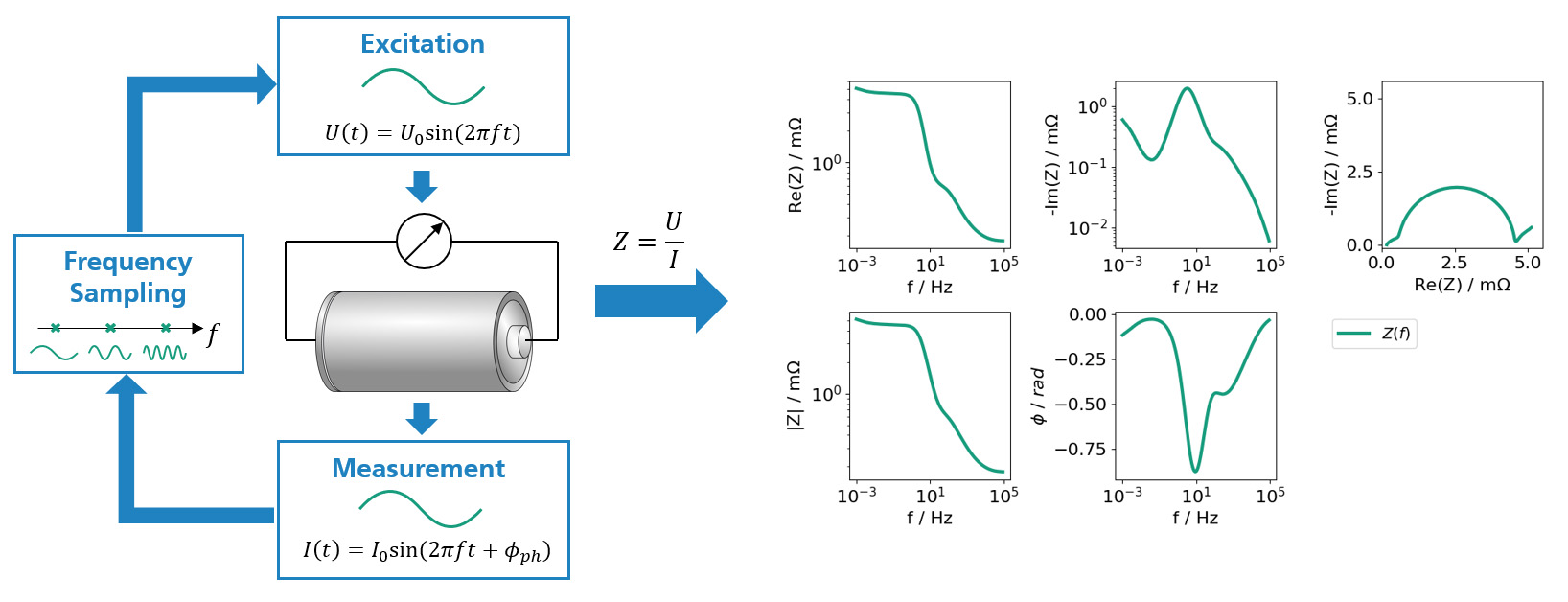
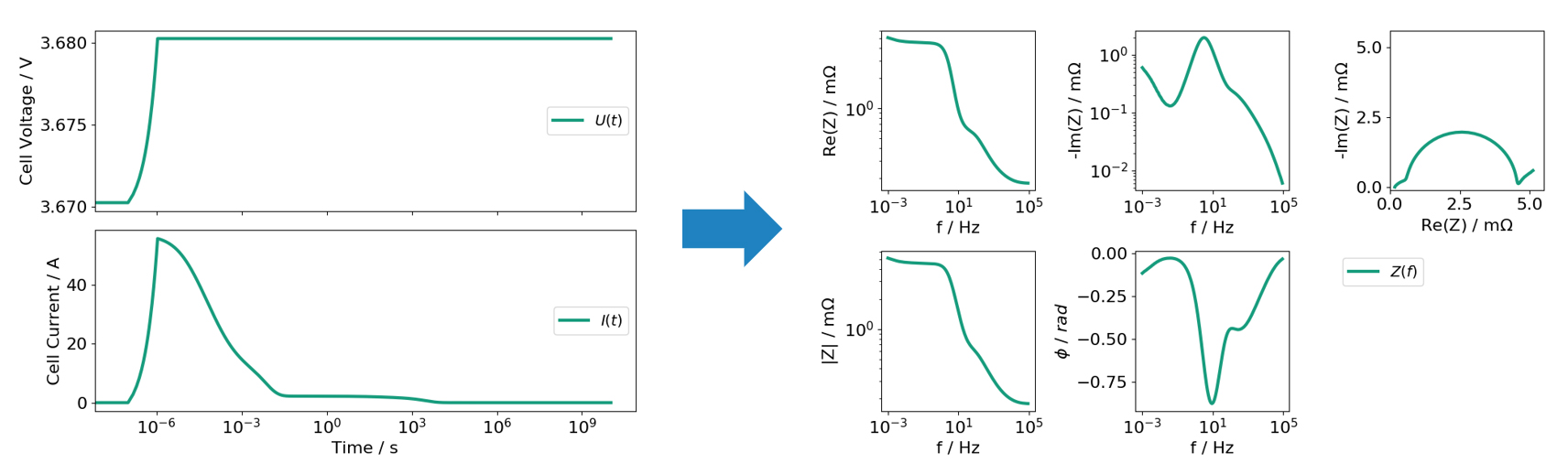
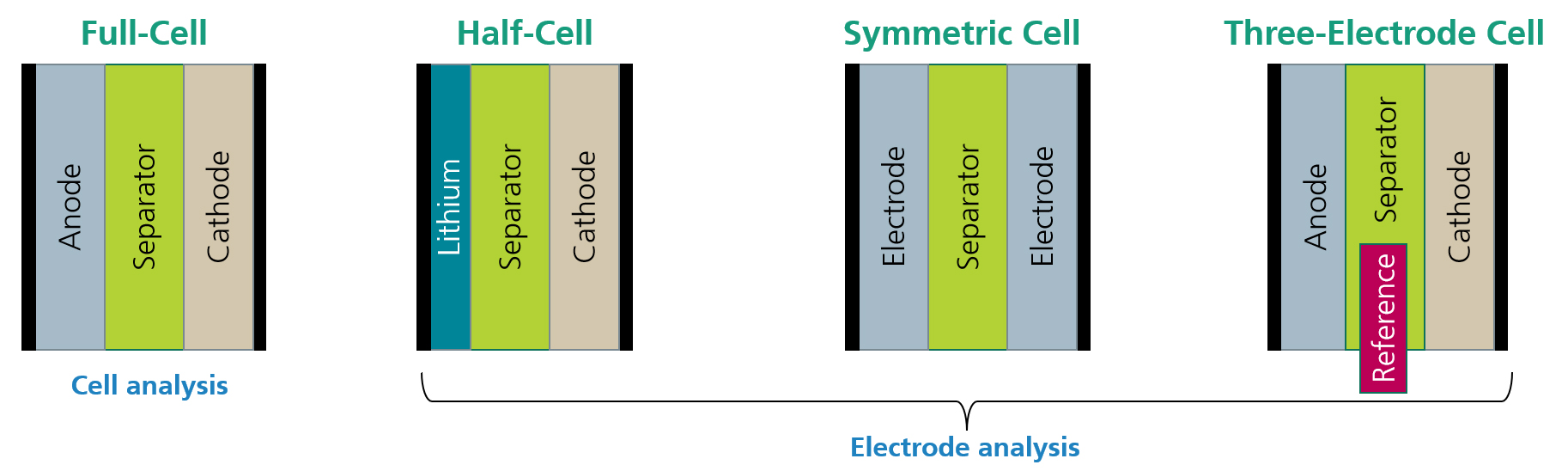
EIS is a popular, non-destructive cell characterization technique for Li-ion batteries. In experiments, EIS is commonly realized by exciting the full battery cell with a small AC voltage or current signal and measuring the current or voltage response of the cell, respectively. Using Ohms’ law allows to compute the impedance with respect to the applied frequency. By repeating this procedure for a variety of frequencies, the impedance spectrum of the cell can be determined step by step.
This spectrum is used to record information over a range of characteristic time scales and therefore contains information about various internal transport processes. By analysing the spectrum in the frequency domain, impedance contributions can be examined as a function of frequency, which allows to draw conclusions about transport processes with corresponding time scales.
This facilitates the determination of the current state of the battery cell, depending on:
- Temperature
- State of charge
- Degradation
- Saturation during electrolyte filling